Health Benefits and Anti-Aging Effects of Licochalcone A
Posted on January 3, 2024 • 13 minutes • 2606 words
Table of contents
Opening Overview
Since ancient times, flora has served as a fundamental resource for nourishment, habitat, and therapeutic remedies. Prior to the advent of contemporary scientific and medical advancements, ancient cultures heavily relied on botanical remedies. Out of approximately 390,000 identified species of plants, a mere 6% have undergone examination for their biological properties. In many developing regions, particularly within Asia and Africa, the utilization of herbal extracts for treating diseases in both humans and animals is still prevalent. Various organic compounds found in plants, such as flavonoids, alkaloids, triterpenoids, saponins, and phenols, are known to facilitate diverse biochemical processes. From these natural substances, numerous pharmaceuticals have been derived. One notable example is Licochalcone A (LCA), sourced from Glycyrrhiza uralensis, which demonstrates significant anti-inflammatory, anti-cancer, anti-obesity, and antibacterial properties. Glycyrrhiza uralensis, the plant from which LCA is derived, has a long history in traditional medicine for its various healing properties, such as replenishing spleen and qi (energy), cooling the body, detoxifying, aiding in expectoration, and alleviating coughs.
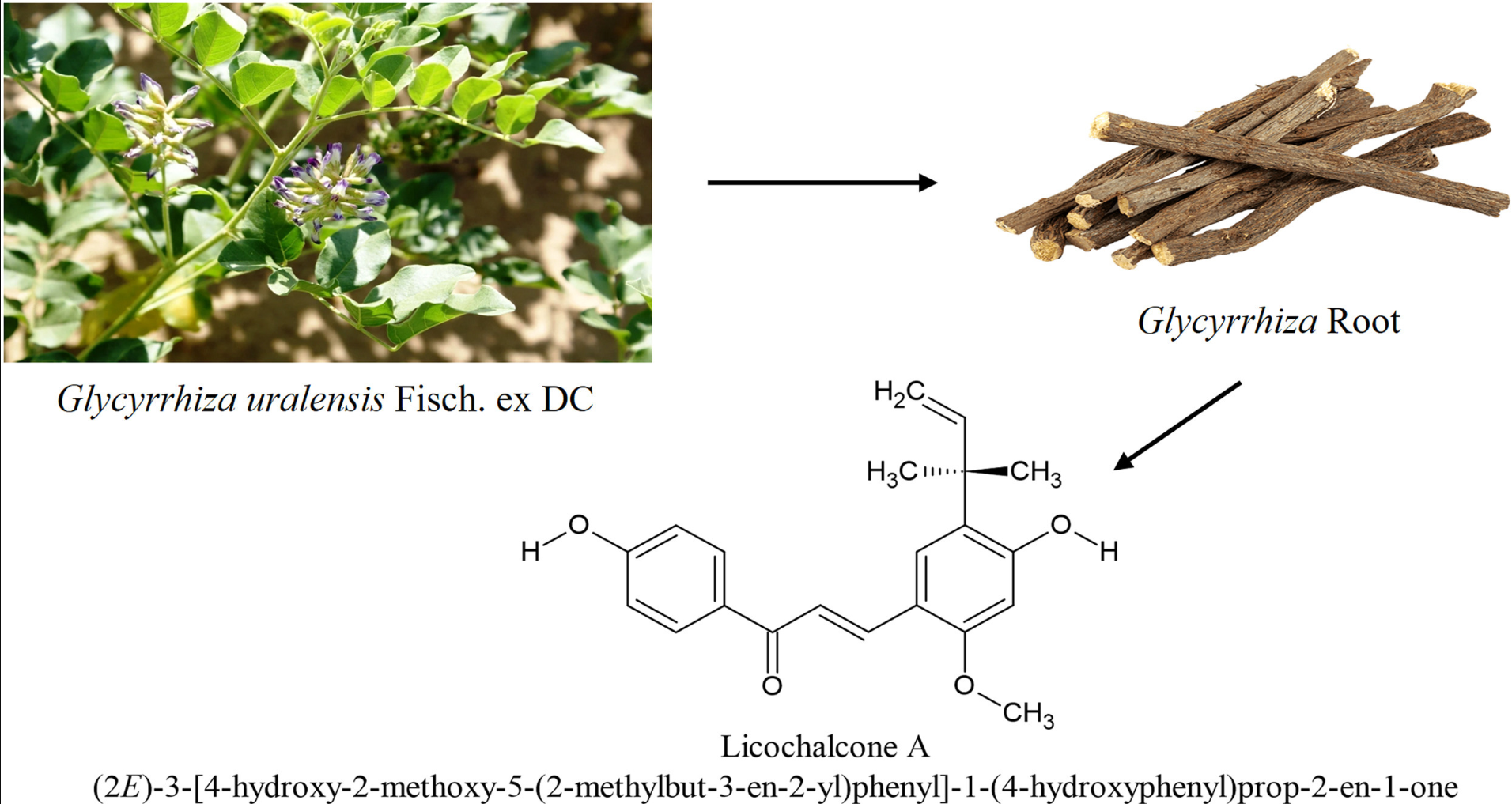
The compound known as (E)-3-[4-hydroxy-3-methoxy-5-(2-methylbutyl-3-ene-2-yl)phenyl]-1-(4-hydroxyphenyl)prop-2-ene1-one, more commonly referred to as Licochalcone A, was first extracted from Glycyrrhiza uralensis roots by Saitoh in 1975. This phenolic chalcone has a molecular structure of C21H22O4. It features two aromatic rings forming its core structure, linked by an α, β–unsaturated α-carbon ketone (as noted by Zhuang et al., 2017). Licochalcone A has a molecular weight of 338.4 g/mol, and it possesses 2 hydrogen bond donors, 4 hydrogen bond acceptors, and 6 rotatable bonds. It has a polar surface area of 66.8° Ų and includes 25 heavy atoms. Notably, it contains two reactive double bonds. One of the unique features of this compound is the unsaturation in its aliphatic side chain, as detailed by Rozmer and Perjési in 2014. The α, β-unsaturation aids in absorbing longer wavelengths of light, facilitating trans-to-cis isomerization, and contributing to ring closure with the hydroxyl function at carbon 4. The thermodynamic stability of the trans (E) isomers over the cis (Z) isomers is also a notable characteristic (Rozmer and Perjési, 2014).
Licochalcone A exhibits a range of biological activities. It has been observed to suppress the expression of the breast cancer resistance protein (BCRP) , and to diminish the cell viability in certain cancer cell lines (Bai et al., 2021). The compound has shown efficacy in alleviating LPS-induced acute liver injury by targeting specific inflammatory cytokines and signaling pathways. It also enhances the expression of antioxidant enzymes in human fetal hepatocytes (X. Chen et al., 2017), and exhibits anti-platelet aggregation properties. In cardiac models, it has been found to improve myocardial function (S. Guo et al., 2016), and in allergic response models, it has shown potential in reducing inflammation. It also demonstrates beneficial effects on the blood-milk barrier in pathological conditions.
Anti aging effects
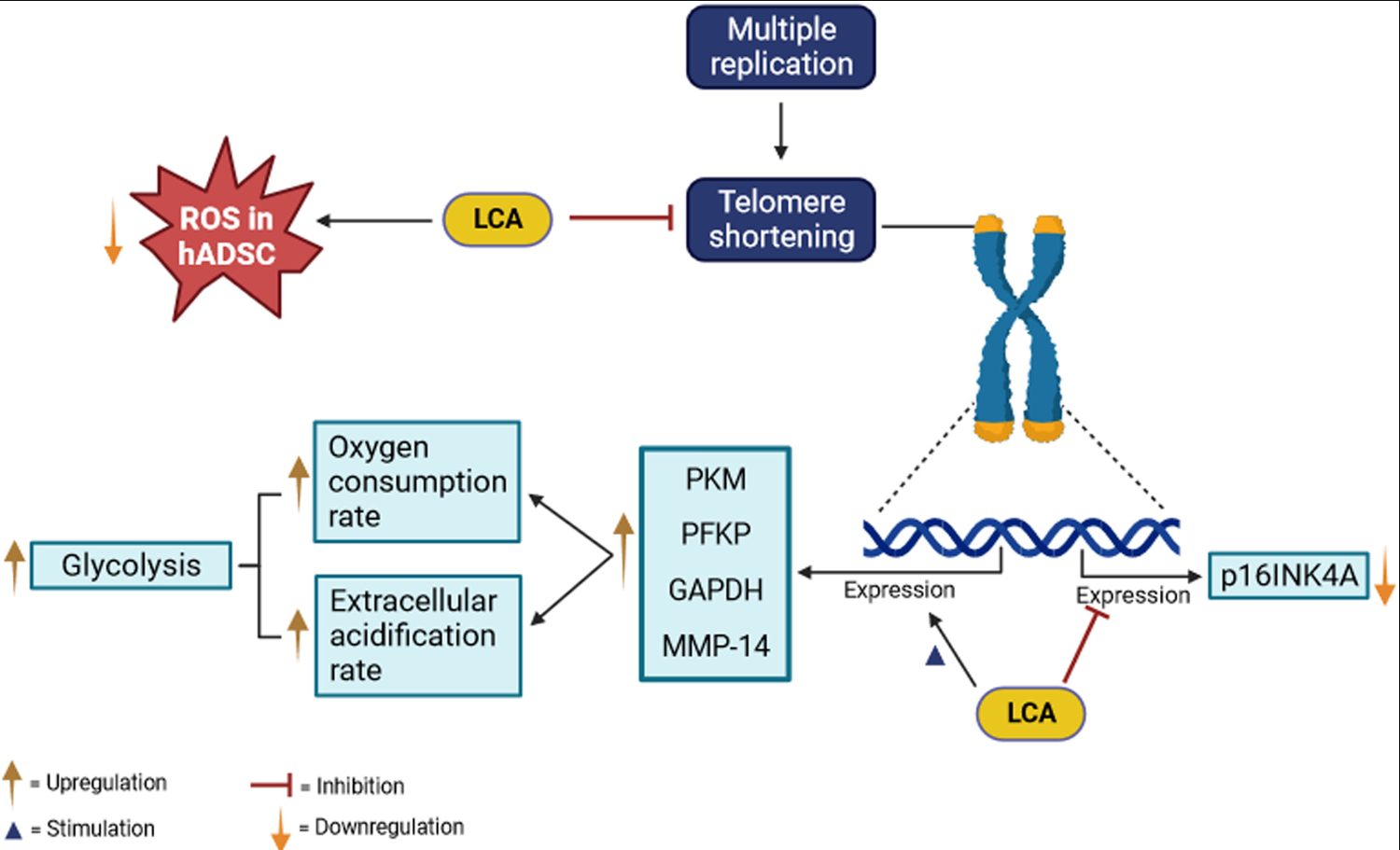
The process of aging involves multiple cellular changes, such as increased reactive oxygen species (ROS), diminishing telomeres, and the activation of certain genes that can lead to cell aging. These changes affect various body functions, including the nervous system and immune response. Lipid profiles also shift during aging, impacting longevity and the emergence of age-related diseases. Factors like genome instability, mitochondrial issues, proteostasis loss, and stem cell exhaustion are linked with aging. Adipose-derived stem cells (ADSCs), known for their self-renewal and high proliferation, can transform into different cell types and contribute to aging processes in animals. Licochalcone A, a compound that affects p16INK4A, a senescence marker, also influences ROS levels and cell proliferation. It has been observed to reduce ROS in human adipose-derived stem cells (hADSCs) and prevent telomere shortening during cell division. Additionally, this compound affects bone marrow adiposity and bone mass in aging mesenchymal stem cells, promoting osteogenic differentiation. Glycolysis activation, crucial for cell growth and energy, is also influenced by Licochalcone A in hADSCs, enhancing cell viability and combating aging effects. This research suggests that Licochalcone A plays a significant role in mitigating aging in human adipose stem cells.
| Anti-aging effects |
|---|
 |
Bone Formation and Strengthening Properties
Bone health is maintained through a careful balance of bone formation and resorption processes. Disruptions in this balance can lead to reduced bone density, increasing the likelihood of osteoporosis. Osteoblasts, which develop from mesenchymal stem cells, play a crucial role in this by mineralizing bone and aiding in calcium deposition, thus contributing to bone strength and structure. Licochalcone A, in concentrations up to 5µM, has shown promise in preventing bone resorption by hindering the development of mature osteoclasts, all while being non-toxic to cells. In osteoporotic rats, a specific dosage of 0.35 mg/kg of a cell-aggregate composite containing Licochalcone A demonstrated enhanced capabilities in bone regeneration and repairing bone defects, showing effectiveness in both laboratory and live settings. This treatment method holds potential in significantly reducing fracture risks associated with bone weaknesses in osteoporotic conditions. Additionally, Licochalcone A has been found to enhance the expression of FAS ligand and stimulate ERK phosphorylation. This process facilitates the inactivation of GSK-3β through a chain of phosphorylation events, leading to the stabilization of β-catenin in bone marrow-derived mesenchymal stem cells. This cascade effect is crucial for improving or restoring the osteogenic differentiation of damaged cells. Moreover, studies on MC4 and C2C12 cells reveal that Licochalcone A induces osteoblast differentiation through the activation of the ERK pathway. Correspondingly, in vivo studies have also demonstrated its role in promoting bone development in Zebrafish and aiding in the formation of mouse calvarial bone.
Platelet activity attenuator
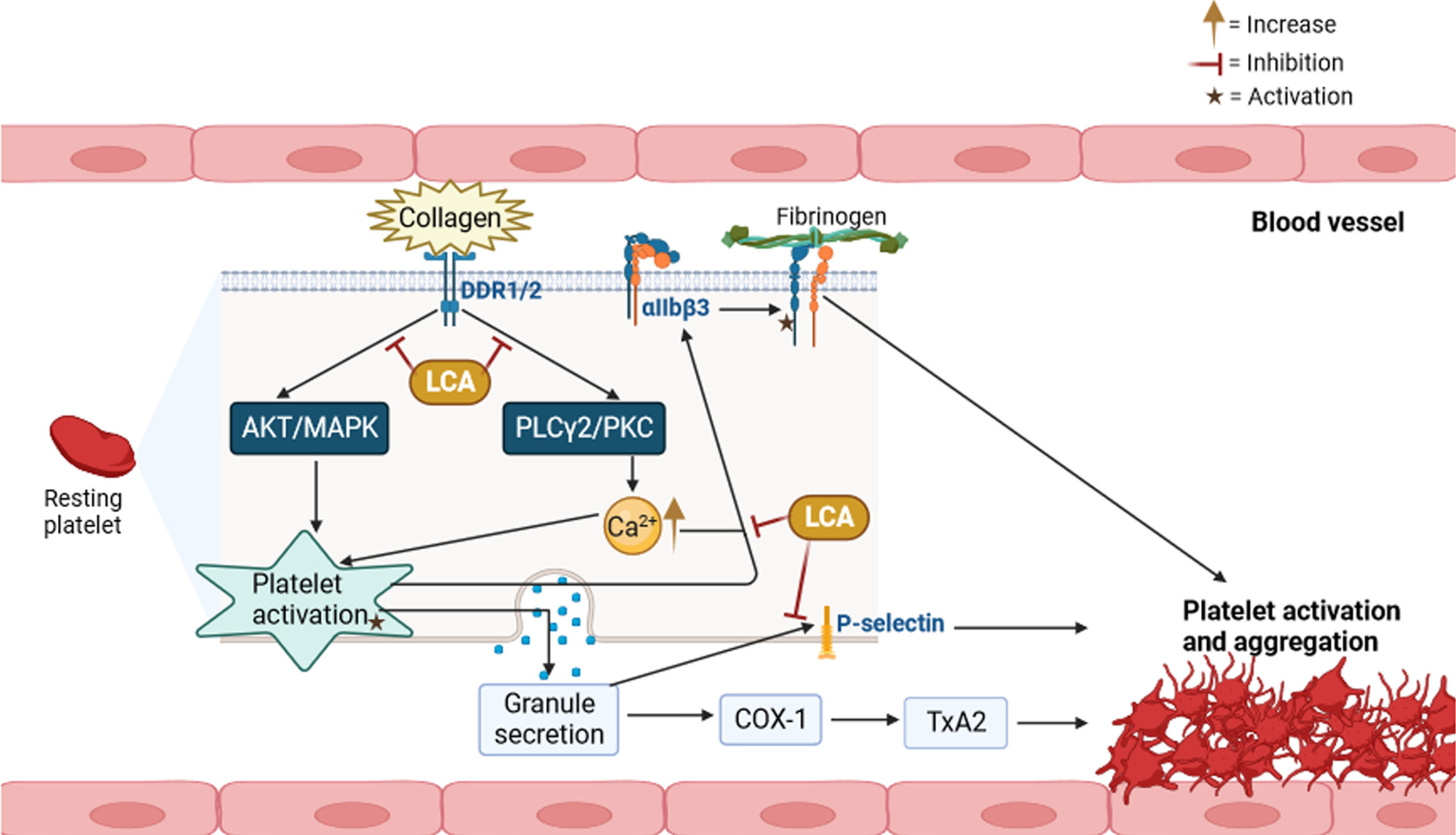
The role of Licochalcone A in preventing cardiovascular diseases, which are often caused by platelet activation leading to high adult mortality rates, is significant. Platelets, originating from megakaryocytes, are crucial in blood vessel health and clot formation at injury sites, maintaining hemostasis. They play a vital role in the development of conditions like atherosclerosis and stroke due to their involvement in blood vessel formation. The process of platelet activation involves the interaction of collagen with specific receptors on platelets, leading to a cascade of events like calcium mobilization and release of granules, essential for clot formation.
However, excessive platelet activation can lead to dangerous clot formation and potential organ failure. To counter this, various antiplatelet agents are used, though they carry a risk of increased bleeding and associated mortality. Licochalcone A, a compound studied both in vitro and in vivo, has shown promise in inhibiting this excessive platelet aggregation. Research indicates that specific concentrations of Licochalcone A can effectively prevent collagen-induced platelet aggregation and related processes, such as ATP release and activation of various signaling pathways. Notably, it achieves this without prolonging bleeding time. Additionally, Licochalcone A has been found to reduce platelet activation by inhibiting the activity of certain enzymes in animal models, further highlighting its potential as a therapeutic agent in cardiovascular disease management.
| Platelets attenuator mechanism |
|---|
 |
Antioxidant properties
The protective qualities of licochalcone A against oxidative damage have been substantiated through various research. In studies measuring its effectiveness, it has shown notable abilities to neutralize free radicals, as indicated by its performance in specific assays where its radical scavenging percentages were recorded at significant levels. Further investigations revealed that licochalcone A’s effectiveness is amplified when combined with certain proteins, demonstrating its potential in diverse applications. Its impact on specific antioxidant protein expressions, which are crucial in mitigating oxidative stress, was observed at certain concentrations, highlighting its potency in these contexts.
Research by Lv and colleagues has shed light on licochalcone A’s capacity to enhance specific antioxidant enzymes in cell models, suggesting its role in promoting cellular defense mechanisms. This enhancement involves complex cellular processes, including the modulation of protein expressions and activities related to antioxidant defense. Additional studies have confirmed licochalcone A’s ability to counteract oxidative stress in various cell types by upregulating essential antioxidant enzymes and inhibiting harmful oxidative processes. These findings point to its potential as a protective agent against oxidative damage, with implications for its use in health-related applications.
Skin protector
Licochalcone A, found in Glycyrrhiza, plays a crucial role in skin health by reducing the release of inflammatory agents like PGE2, leukotriene B4, Il-6, and TNF-α, thereby safeguarding against skin inflammation. It is particularly effective in diminishing sunburn erythema, a common result of ultraviolet exposure, as evidenced in tests using a Glycyrrhiza aqueous extract with 0.05% licochalcone A concentration. This compound also hampers the activity of tyrosinase, an enzyme pivotal in melanin production, thus aiding in the prevention of hyperpigmentation.
Further studies highlight its benefits in enhancing skin barrier function and hydration while reducing the presence of S. aureus, particularly beneficial for individuals with atopic dermatitis. Additionally, licochalcone A has been observed to bolster the cellular defense against oxidative damage in human fibroblasts, attributed to its antioxidant properties and the activation of Nrf2, which boosts the expression of enzymes like HO-1 and GCLM.
In comparison to hydrocortisone, a standard treatment for various skin conditions, licochalcone A has demonstrated similar efficacy in managing atopic dermatitis with a reduced risk of relapse. Its potential in skin cancer prevention is also noted, particularly its inhibitory effects on COX-2 generation and AP1 transcriptional activity in response to solar ultraviolet exposure. Furthermore, licochalcone A shows promising results in mitigating ROS formation induced by visible light, an area where traditional UV filters and SPF products have limited effectiveness.
Neuroprotection
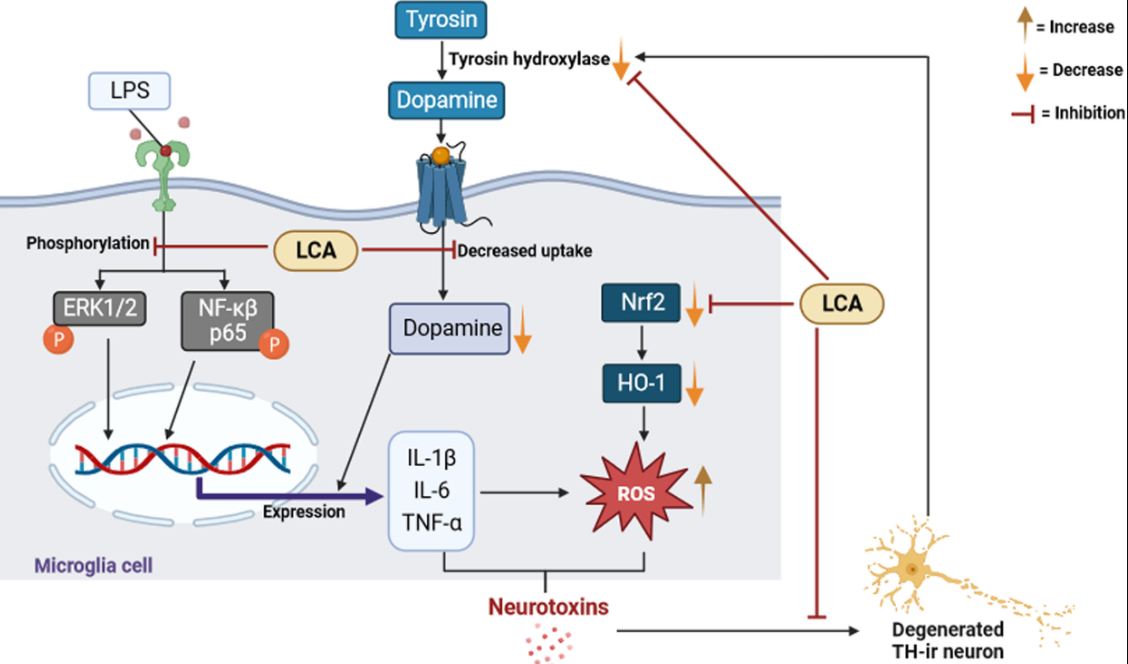
Neuropathic pain often arises from damage to neurons, which triggers a cascade of inflammatory responses involving microglial cells in the brain and spinal cord. These responses are marked by the release of various chemokines and cytokines. Following a peripheral nerve injury, an immune response is activated, leading to pain due to the activation and proliferation of microglia in the spinal cord. This process is closely linked with the development of pain hypersensitivity. Microglial activation involves changes in cellular characteristics like shape, phagocytosis, and migration. Research has shown that the administration of licochalcone A in certain dosages effectively reduces neuropathic pain in rat models.
Parkinson’s disease, primarily affecting older adults, is characterized by symptoms such as rigidity and slow movement. It is marked by the degeneration of dopaminergic neurons in a specific brain region. The disease’s progression is closely linked to neuroinflammation, where overly activated microglial cells release various proinflammatory mediators, contributing to neuronal damage. Inflammatory models resembling Parkinson’s disease show extensive microglial activation and loss of dopaminergic neurons. Licochalcone A has been found to inhibit the production of proinflammatory mediators in certain cell models and promote dopamine uptake, suggesting its potential in mitigating Parkinson’s disease symptoms.
Perinatal hypoxic-ischemic encephalopathy, a significant cause of neonatal mortality and long-term disability, results from inadequate blood flow and oxygen to the brain in newborns. This condition is associated with oxidative stress and neuroinflammation. Licochalcone A has shown potential in reducing oxidative stress markers and pro-inflammatory cytokines in neonatal rat models of this condition. Additionally, it has shown neuroprotective effects against neuronal injury in these models. Furthermore, licochalcone A has demonstrated promising results as a potential anticancer agent for certain brain tumors, indicating its ability to induce apoptosis in cancerous cells with minimal toxicity to normal cells.
| Neuroptoection mechanisms |
|---|
 |
Adverse effects
In studies involving BALB/c mice, Licochalcone A (LCA) demonstrated no adverse effects on the viability or proliferation of HFF host cells, even at concentrations up to 9μg/mL. This suggests its relative safety for use in research exploring its anti-parasitic properties. While licorice root extract, a component of the Chinese medicine Yokukansan used for Alzheimer’s disease, has shown some adverse effects like hyperkalemia in elderly patients, LCA exhibits distinct characteristics. For example, LCA’s antifungal capabilities against Candida albicans were notable, although higher doses (≥ 20μg/ml) reduced the viability of oral epithelial cells. Further, human renal cortical tubule epithelial cells and U-87 MG cell lines maintained good viability even at 50μM LCA concentrations over 48 hours. Oral administration of LCA up to 1,000 mg/kg in rats did not yield any visible toxicity signs, reinforcing its low-toxicity profile.
Additionally, macrophage studies revealed no toxic effects from LCA across various concentrations. Its role in reducing the side effects of cisplatin in cancer treatments, particularly in minimizing damage to the kidneys and liver, has been promising. In contrast to its benign effects on mammalian host cells, LCA induced significant changes in adult Schistosomamansoni worms, with an 87.5% viability rate even at 400μM concentrations. However, certain cell types like osteoblasts and RAW 264.7 cells showed reduced viability at higher LCA dosages. Interestingly, human gastric epithelial cells tolerated high LCA levels well, while it proved toxic to BGC-823 cancer cells. Similarly, normal human oral keratinocytes remained unaffected by LCA treatments that were detrimental to FaDu cells.
Moreover, LCA’s impact on human monocytes was minimal at lower concentrations, with notable effects on mitochondria only at significantly higher levels. This selective impact was also observed in its treatment of parasitic cells, where low concentrations caused substantial damage. Reiterating its safety, oral administration of LCA in rats at high doses reported no toxicity. Its non-toxic nature was further evidenced in studies involving non-tumor cell lines like HBE and NL20. However, moderate cytotoxicity was observed in peritoneal murine macrophages, and BEAS-2B human cell lines experienced low toxicity in a dose-dependent manner. Lastly, LCA’s effectiveness against rheumatoid arthritis without affecting body weight even at high doses further exemplifies its low toxicity and potential as a chemotherapeutic agent.
LCA as a therpeutic agent
Licochalcone A (LCA) serves as a complementary drug in traditional eastern medical practices. It has been found to reduce the viability of certain cancer cells by triggering cell death. Furthermore, its effectiveness against S. mansoni, particularly when used in nano-formulations like LCA-loaded solid lipid nanoparticles, highlights its potential in nano-medicine. LCA derivatives act as potent inhibitors of certain enzymes from M. tuberculosis, demonstrating effectiveness even at low doses.
In the context of oral cancer, LCA shows promise as a preventative agent, effectively hindering the spread of specific carcinoma cells by blocking their adhesion, movement, and invasive capabilities. It also counteracts the effects of certain inflammatory agents on chondrocytes in human osteoarthritis. Interestingly, LCA can also overcome drug resistance in tumors, particularly those resistant to multiple drugs due to ABCG2 mediation, by reversing resistance to certain drugs at slightly higher concentrations.
LCA has been identified as a potential agent against skin cancer caused by solar UV rays, primarily by inhibiting COX-2 expression induced by solar ultraviolet. Its ability to work synergistically with antimalarial drugs like artemisinin, enhancing their effectiveness, is another notable feature. Moreover, LCA has been observed to interfere with the metabolic actions of various enzymes responsible for drug metabolism, suggesting a broad-spectrum inhibitory potential.
However, LCA’s interaction with other drugs is a critical consideration, especially given its inhibitory effect on several human cytochrome isozymes. While a crude licorice extract shows more potency than LCA alone, possibly due to additional organic compounds, the potential for herb-drug interactions remains a concern.
In obesity treatments, LCA has emerged as a promising candidate for inhibiting pancreatic lipase, thus potentially reducing dietary fat absorption. This suggests its utility in weight management therapies, pending further animal trials. Additionally, LCA can enhance the bioavailability of certain drugs like nifedipine, a calcium channel blocker used for hypertension, by inhibiting specific enzymes and proteins in the intestine, indicating its potential in combined drug therapies.
Closing words
Licochalcone A, a flavonoid distinguished by its unique chalcone structure, exhibits considerable pharmacological promise for treating various health issues. This review has brought to light its potential in several key areas, such as mitigating platelet aggregation, addressing skin conditions, alleviating oxidative stress, offering neuroprotection, and serving in anti-aging treatments. Its favorable safety profile enhances its appeal for new therapeutic developments. Nonetheless, further exploration into its safety and effectiveness, particularly concerning drug interactions, is warranted. Licochalcone A continues to be a compelling subject for research in the realms of natural products and pharmaceutical advancements.
Share
Tags
Counters

